<<back
Lesson Plan PHILIPPINES
F. Philippines
F.1. Water and environmentally sustainable development
F.1.1. Overview and Background Information
Water is the most abundant, most widely distributed, and most commonly used of all chemicals. No living things – plant or animal – can survive without it. But the unique property of water has been altered due to people’s carelessness and irresponsibility of dumping their wastes to water sources thus making it polluted.
Are there ways of preventing water pollution? Municipal water treatment plants utilize several processes before they discharge water to the water users. The basic method involves removal of suspended particles, aeration, flocculation, sedimentation, and chlorination.
Oil spill is a growing problem regarding water pollution, particularly to coastal wildlife. This lesson will give insights and information to the learners in removing and cleaning an oil spill.
F.1.2. The Lesson Plan
Subject Matter area |
Chemistry |
Time duration |
Topic |
Water on Earth |
|
Sub-topic |
Ways of Removing Water Pollutants? |
|
Grade Level |
3 |
|
Specific concepts covered in the lesson |
- Ways of Cleaning Oil Spills
|
|
Human values addressed |
- Respect for life
- Sense of responsibility toward the environment
- Self-involvement
|
|
Lesson objectives |
By end of the lesson, learners should be able:
- To identify ways of removing oil spills in seas and oceans.
- To perform simple ways of removing oil spills in seas and oceans
- To appreciate and put into practice our duties and responsibilities of taking care of our water resources
|
|
Materials needed |
- Activity materials: plastic basin, wooden stick, paper plate, rubber bands, chicken feathers, 3 hard-boiled eggs, dishwashing detergent, used motor oil, hypodermic syringe, rags/tissue paper, spill incident wastes
- song lyrics
- pictures/video clips of an oil visual aids
|
|
Teaching-Learning Steps: |
120 min |
Introductory or Motivational activities |
- Prayer
- Classroom Management
- Review of the Past Lesson
- Let the learners view a video clip of the oil spill incident happened in Guimaras Island, Philippines.(If video clips are not possible, you can use newspaper pictures of the oil spill incident; (See Attachment 6.F.1.1)
- Ask the learners what they have observed in the video clips/pictures. Use the table below to facilitate learners’ ideas.
|
10 mins
15 mins |
Table 6.F.1.1. Observation list on video clip about oil spills
What is the cause of the problem? |
Which/Who will be affected by the problem? |
What solution they propose to solve the problem? |
|
|
|
|
|
|
|
|
|
|
|
|
- Also show a picture of an oiled bird due to the oil spills.(See Attachment 6.F.1.2) Ask the class the harmful effects that the bird may from the oil spills.
|
|
Pre-activity |
- Ask the class to work in groups and perform the activities to clean oil spills. The teacher is expected to set guidelines and standards in doing the experiment. He/she is also expected to briefly review the safety precautions and laboratory techniques to be observed in doing experiments.
|
10 mins |
Lesson proper
|
Step 1: Activity Proper
- Perform the activity entitled “Ways of Cleaning Oil Spills”. (See Attachment 6.F.1.3) Make sure to remind the learners to answer the questions that follow.
|
20 mins |
Step 2: Post Activity Proper
- Ask the class to form their respective groups again. Ask the learners to share ideas and insights to answer the questions given in the worksheet.
- Ask the leader of the class to do the reporting of their results.
|
20 mins |
Additional Information:
- Oil is less dense and insoluble in water, the oil floats on water.
- Oil spills can be very harmful to marine birds and mammals, and they can also harm fish and shellfish.
- When there is too much oil on the water’s surface, sunlight cannot penetrate the water layer, then plants in the water cannot manufacture food.
- There are other ways of removing oil spills aside from the methods you implemented in your experiment. Burning is one way but it is dangerous.
|
10 mins |
Generalization
- There are mechanical methods of cleaning oil spills in the water such as using absorbents, skimmers, and dispersing agents.
- Absorbents like sponges and feathers may be used to absorb oil. Other absorbents are straw, hay, sawdust, corncobs, or even volcanic ash. (see Attachment 6.F.1.4)
- Skimmers use oil-attracting materials that can blot oil from the water surface. The oil is the squeezed out or scraped off into a task.
- The use of dispersing agents like detergents can be also used to clean oil spills.
|
3 mins |
Linkage with human values: |
Water is a natural resource that is very important to our daily lives. It is a necessity. Just like the importance of other people to our life. As the saying goes, “No man is an Island”. We can’t live alone; we need others to survive as we need water in our lives. So in order for us not to be alone, we have to know how to keep and to care for them. We should know how to respect them as we respect and conserve our water resources. |
2 mins |
Closure |
Ask the class to have a reflection on the lyrics of the song “Anak ng Pasig” of Geneva Cruz (Attachment 6.F.1.4). (If the song is not available, you can choose other songs or poems related to water pollution. Ask them to get the message conveyed by the song. |
15 mins. |
Assessment / Evaluation |
Observation and test (See Attachment 6.F.1.5) |
15 mins |
Attachment 6.F.1.1
PICTURES OF THE GUIMARAS ISLAND
INCIDENT in the Philippines


Attachment 6.F.1.2
An Oiled Bird


Attachment 6.F.1.3
Learners Activity Worksheet
Name of learner : _________________________________________
Year level : _________________________________________
Subject Matter Area : _________________________________________
Topic : _________________________________________
Title of Activity : Ways of Cleaning Oil Spills
Objective(s) of the Study:
- to describe the ways to clean oil spills
Materials
plastic basin wastes used motor oil
paper plate wooden stick hypodermic syringe
chicken feathers rubber bands rags/tissue paper
dishwashing detergent 3 hard-boiled eggs
Process and Steps:
Process |
Steps |
Note: time, etc. |
Preparation for learners to participate in the activities
- Group work set up
- Check learning materials
|
- Learners in groups with about 5-6 members each group
- Choose leader of group
- Checking your materials mentioned above + activity worksheet + evaluation sheet
|
- In classroom or laboratory
- 10 minutes
|
Activities:
Step by step guidance for learners (incl. questions, and expected answers)
- Group work to clean oil spills |
The tasks : cleaning oil spills
- Doing experiments as given in the following pages
- Observations and Inferences
- Answering questions
- Generalization
|
- in laboratory
- 25 minutes
|
Expected Results
- The results of experiment
- Presentation |
Present the result of experiments |
- in classroom
- or laboratory
- 20 minutes
|
Procedures
A. Using feathers
- Fill a basin half-full of water. Pour enough used motor oil to cover the water’s surface.
- Place the feathers in the water. Leave the feathers in the oil for about 10 minutes. Try to push the feathers into the water.
- Remove the feathers after 10 minutes. Note the changes in the feathers. How will you remove the oil from the feathers?
- Wipe the oil from the feathers with rags or tissue paper. Describe what happened to the feathers afterwards.
B. Using hard-boiled eggs
1. Put the three hard-boiled eggs (with shells on) into a basin with the oil-water mixture.
2. Remove one egg from the basin after 20 minutes. Remove another egg after 40 minutes, and then the third egg after 1 hour. Describe the appearance of the three eggs.
3. Carefully wipe the eggshells with rags or tissue paper. Describe what happened to the eggshells.
4. After wiping the oil from the eggs, crack and remove all the egg shells. Observe closely the egg whites. Did oil get into the egg?
C. Using paper
- Spread out tissue papers on the surface of oil in the basin. Keep the tissue papers there for about 5 minutes and then remove them and spread out on a paper plate.
- Spread out a large rubber band on the oil’s surface. Note down your observations. Compare them with those on feathers and tissue papers.
- Use a hypodermic syringe to remove the contained oil. How much of the oil was removed?
D. Using detergent solution
1. Add several drops of detergent dishwashing liquid into the remaining water-oil mixture in the basin.
Guided Questions:
A. Using feathers
1. What happened to the feathers when these were placed in water covered with oil?
2. How much oil do you think was removed? Can wiping with rags or tissue paper remove oil?
3. What do you think happens to birds that come into contact with oil spills in oceans and seas?
B. Using hard-boiled eggs
1. Can wiping the eggs with rags or tissue paper remove oil? Compare the results of wiping on feathers and eggshells.
2. Did oil get through the egg shells? Explain your answer.
3. If oil got through the egg shells, can it be removed?
C. Using paper
1. What is the effect of laying out tissue papers on the oil’s surface?
2. What is the effect of laying out a large rubber band on the oil’s surface? Will removing oil with this method remove more oil?
3. Can the hypodermic syringe remove most of the oil? What is the disadvantage of this method?
D. Using detergent solution
1. Describe what happens to the oil.
2. Is detergent liquid another technique of removing oil?
3. Which of the methods is best for removing the oil? Which is best for keeping the oil in place?
4. Although detergent solution can remove oil by breaking its surface tension, what other undesirable effect does it introduce into the water?
Attachment 6.F.1.4
ANAK NG PASIG
Artist: GENEVA CRUZ
Album: UNKNOWN
Ako'y umusbong sa tabi ng Pasig
Nagisnan ang ilog na itim ang tubig
Lumaking paligid ang bundok na umuusok
Langhap na langhap ang amoy ng basurang bulok
Ganyan ang buhay ng anak ng Pasig
Pa-swimming swimming sa itim na tubig
Playground lang ang bundok ng basura niyo
Musika'y ugong ng kotse at bangka niyo
Chorus:
Anak ng Pasig naman kayo
Kalat doon, kalat dito
Natakpan na ang langit kong ito
Nilason din ang Ilog ko
Anak ng Pasig naman kayo
Tapon doon, tapon dito
Di niyo alam ang tinatapon niyo
Ay bukas ko at ng buong mundo
Akala ko'y ganoon talaga ang mundo
Hanggang makakita ako ng lumang litrato
Di maniwalang Pasig rin ang tinitingnan ko
Kaibigan ano ang nangyari ditto
Attachment 6.F.1.5
Assessment/Evaluation
A. Direction: Write true if the statement is correct and false if it is wrong on the space provided before the number.
________ 1. Oil floats in water because oil is denser and soluble in water.
________ 2. Oil spills can be very harmful to marine birds and mammals, and they can also harm fish and other aquatic organisms.
________ 3. Burning is the safest way of removing an oil spill.
________ 4. Skimmers use oil-attracting materials that can blot oil from the water surface.
________ 5. Only the officials should help in the care and conservation of earth’s water to ensure the availability of good quality water for the use of the world’s growing population.
B. Draw a poster to show your appreciation and concern about having a clean and safe water.
F.2. Water, social equity and human dignity
F.2.1. Overview and Background Information
Knowing that man cannot live without water and that the amount of water in our universe is not likely to increase, we can understand why we should use our water resources wisely.
But unfortunately, many people are careless. They throw their wastes directly into bodies of water, thinking that wastes will all be dissolved by water and the water will carry the wastes as it moves along. They fail to understand that the solid and liquid wastes that they dump in drains or drop on the ground eventually end up in bodies of water.
When water becomes unfit for its intended use, it is considered polluted. Water pollution occurs when a body of water is adversely affected due to the addition of large amounts of materials to the water. These are called water pollutants.
We should be careful on all the substances added or removed from the water which adversely affect their use for domestic, commercial, industrial, agricultural, recreational, or other legitimate purposes.
F.2.2. The Lesson Plan
Subject Matter area |
Science |
Time duration |
Topic |
Water Pollution |
|
Grade Level |
3 |
|
Specific concepts covered in the lesson |
- How Pollutants Alter the Properties of Water
|
|
Human values addressed |
- Protecting oneself and the environment
- Consciousness on water pollution
|
|
Lesson objectives |
By end of the lesson, learners should be able:
- To identify the different compounds that alter the properties of water
- To describe how water reacts when mixed with these compounds
- To identify the chemical properties of water through the chemical changes it undergoes.
|
|
Materials needed |
- Activity materials: 8 small clear bottles, water, 8 plastic tablespoons, waste cooking oil, marker pen, vinegar, table salt, food coloring, ashes, baking soda, leaves or wood chips.
- pictures of polluted and unpolluted water source
|
|
Teaching-Learning Steps: |
120 min |
Introductory or Motivational activities |
- What are the properties of a water resource that make it uniquely suited to support life? Show pictures of non-polluted water resource/s.(See Attachment 6.F.2.I)
- Compare a non-polluted water resource with a polluted water resource/s. Show pictures of polluted water resource/s.(See Attachment 6.F.2.2)
|
10 mins
|
Pre-activity |
- Divide the class into groups and prepare all the materials to be used in the activity. If there are not enough equal-sized bottles available, you may use transparent drinking glass instead.
- Set standards to be employed during the activity proper.
- Briefly review on the precautions and laboratory techniques.
|
15 mins |
Lesson proper
|
Step 1: Activity Proper
- Perform the activity entitled “Water Mix-Mix”. (See Attachment 6.F.2.3
- Make sure you remind the learners to answer the questions followed.
|
35 mins |
Step 2: Post Activity Proper
- Which observations showed that materials can
- change the color of water?
Answer: food coloring, ashes, and used cooking oil change the color of water, vinegar, table slat, and baking soda make the water less clear or cloudy depending on the amount added; oil forms a layer on water.
- change the smell of water?
Answer: The change in smell of water is very distinct in vinegar.
- make the taste of water better? bad?
Answer: Vinegar and salt drastically change the taste of water. Food coloring, if flavored will change the taste of water too.
- change the way water feels?
Answer: Oil can make water feel slippery and sticky. Ashes and leaves can make water feel gritty because of the presence of very tiny solid particles.
- produce new materials with water?
Answer: Water reacts with baking soda to form bubbles of carbon dioxide gas. Some components of ashes like potassium oxide and sodium oxide react with water to form bases such as potassium hydroxide and sodium hydroxide.
- Name some other materials that can
- change the color of water.
(Answers may vary)
- make the smell of water better, awful, or repulsive.
(Answers may vary)
- make the taste of water better; bad.
(Answers may vary)
- change the way water feels.
(Answers may vary)
- produce new materials with water.
(Answers may vary) |
20 mins |
|
Additional Information:
- Water when pure is colorless, transparent, odorless, tasteless.
- Water is a chemical, it reacts with other substances producing new materials.
- Water is called the “universal solvent” because it can dissolve more substances than any other liquid.
|
5 mins |
|
Generalization
- Some materials like salts, acids, and bases are soluble in water, thus changing the properties of water.
- Some materials not only dissolve but also react with water producing new materials.
|
5 mins |
Linkage with human values: |
Pollutants such as organic wastes, garbage, oil, chemicals, and other toxic substances makes the water unfit for its intended purpose. Due to man’s carelessness and improper waste management, our bodies of water have become polluted. Just like the wrong virtues and values that most people acquire from outside sources, it will also pollute one’s personality and makes someone useless and worthless. Do not let these wrong virtues be added and absorbed by our life. |
|
Closure |
End the session by asking learners to give their insights on the following statement made by Rene Dubois, a world renowned biologist.
“We will destroy our lives by producing more useless, destructive energy to make more and more needless things that do not increase the happiness of people.” |
15 mins. |
Assessment / Evaluation |
List down chemical substances that can alter the properties of water.
- home
- markets
- hospitals
- factories
- farms
|
15 mins |
Attachment 6.F.2.1
A non-polluted water source



Attachment 6.F.2.2
A Polluted Water Resource

Attachment 6.F.2.3
Learners Activity Worksheet
Name of learner : ___________________________________________
Year level : ___________________________________________
Subject Matter Area : ___________________________________________
Topic : ___________________________________________
Title of Activity : How Pollutants Alter the Properties of Water
Objective(s) of the Study:
- to study pollutants that alter the properties of water
Materials
8 small clear bottles water
8 plastic tablespoons waste cooking oil
marker pen vinegar
table salt food coloring
ashes baking soda
leaves or wood chips
Process and Steps:
Process |
Steps |
Note: time, etc. |
Preparation for learners to participate in the activities
- Group work set up
- Check learning materials
|
- Learners get into groups
- Choose leader of group
- Checking your materials mentioned above + activity worksheet + evaluation sheet
|
- In classroom or laboratory
- 15 minutes
|
Activities:
Step by step guidance for learners (incl. questions, and expected answers)
- Group work to study some pollutants that can change the properties of water |
- The tasks : investigating which pollutants that can change the properties of water.
- Doing experiments as following procedures.
- Observations and Inferences
- Answering questions
- Generalization
|
|
Expected Results
- The results of experiment
- Presentation
|
Present the result of experiments |
- in classroom
- or laboratory
- 20 minutes
|
Procedure
- Label the bottles numbers 1-8. Do the same to the spoons. Assign one spoon to a bottle.
- Describe the properties of water and all the materials given. Enter your descriptions in Table 6.F.2.1.
- Half-fill each bottle with water. Bottle # 1 will serve as the control. Nothing will be added to it.
- Add four tbsp of vinegar to bottle # 2 and stir with the spoon. Observe the effects of the materials on the observed properties of water. Enter your observations in Table 6.F.2.2.
- Add 4 tbsp of waste cooking oil to bottle # 3, stir and observe.
- Add 2 tbsp of table salt to bottle # 4, stir and observe.
- Add 3 tbsp of baking soda to bottle # 5, stir and observe.
- Add 2 tbsp of food coloring to bottle # 6, stir and observe.
- Add 3 tbsp of ashes to bottle # 7, stir and observe.
- Add 2 tbsp of leaves to bottle # 8, stir and observe.
- Complete table 2 with your observations.
Data
Table 6.F.2.1. Properties of materials before mixing with water
Properties |
Observations |
water |
vinegar |
cooking oil |
table salt |
baking soda |
food coloring |
ashes |
leaves |
Color |
|
|
|
|
|
|
|
|
Smell |
|
|
|
|
|
|
|
|
Taste |
|
|
|
|
|
|
|
|
Feel |
|
|
|
|
|
|
|
|
Table 6.F.2.2. Properties of materials after mixing with water
Properties |
Observations |
water |
vinegar |
cooking oil |
table salt |
baking soda |
food coloring |
ashes |
Leaves |
Color |
|
|
|
|
|
|
|
|
Smell |
|
|
|
|
|
|
|
|
Taste |
|
|
|
|
|
|
|
|
Feel |
|
|
|
|
|
|
|
|
Guide Questions:
1. Which observations showed that materials can
- change the color of water?
- change the smell of water? Describe the change in smell.
- make the taste of water? bad?
- change the way water feels?
- produce new materials with water?
2. Name some other materials that can
- change the color of water.
- make the smell of water better; awful or repulsive.
- make the taste of water better; bad
- change the way water feels.
- produce new materials with water.
3. Name some of the good and bad effects of the addition of substances to water.
4. Relate your observations to your daily activities by identifying where you have seen similar occurrences.
Generalization:
____________________________________________________________
____________________________________________________________
____________________________________________________________
F.3. Water for health, sanitation and recreation
F.3.1. Overview and Background Information
The lesson is about the importance of water to our daily living. More so, it extends to the value of water conservation.
The lesson teaches us the value of water conservation through taking care of our forest as well as planting more trees. It also teaches the proper way of using water in our daily life.
The lesson is in MAKABAYAN (Social Studies). It is being integrated with other subjects such as Science and Health as well as Values Education. It is for grade III learners and they are expected to have learned about the basic needs of man including water.
F.3.2. The Lesson Plan
Subject Matter area |
Makabayan |
Time duration |
Topic |
Water as a Basic Need of Man |
|
Grade Level |
3 |
|
Specific concepts covered in the lesson |
- Water is one of the basic needs of man. We should learn to use it wisely.
- Water from the rivers, lakes, and seas gives us many things. The food that we eat like fish, crab, shells and shrimps come from these water sources.
- We use water for cooking, drinking, washing, and even taking a bath.
- Waterways are used to transport people and goods from one place to another.
- We need to conserve water to continually enjoy the benefits that it gives.
- We should keep water sources clean.
- Planting more trees help maintain the water cycle and preserve water sources.
|
|
Human values addressed
Human values addressed |
- Water is one of the basic needs of man without which we will not survive. As such, we need to conserve it and always ensure that it is clean.
- Learn about the different ways of using water wisely and practice these in our daily lives.
- Whatever we take away, we should give back. We use water everyday; in return we should conserve it to fully enjoy the bounty of nature.
|
|
Lesson objectives |
To explain the importance of water as one of the basic needs of man. Enumerate the different ways by which we can conserve water.
To appreciate the value of water and put into practice the different ways of conserving water.
To make a poster or slogan on conserving water. |
|
Materials needed |
- Jumbled letters for the puzzle games
- A composition about water (maybe sang to the tune of “Paruparo’ng Bukid” or read as a poem; written in Filipino and English)
- Pictures on the uses of water
|
|
Teaching-Learning Steps: |
120 min |
Introductory or Motivational activities |
- Preparatory activities: prayer, greetings, checking of Assignments
|
5 mins |
|
Review
- Form a word from the jumbled letters. The words formed are taken up from the last lesson.
ODOF – the most important basic need of man. Without it, man will die.
IOLNAMARTINUT – cause by lack of nutritious food to eat.
- Why do we need food?
- What kind of food should we eat?
- What should we do so that we will always have fruits and vegetables?
|
5 mins |
|
Motivation
The learners will be asked to study the composition, entitled “Tubig ay Tipirin” (Conserve Water as on Attachment 6.F.3.1). This maybe sang to the tune of “Paruparo’ng Bukid”, a Philippine folksong, or be read as a poem. The teacher may also use any song related to water. |
15 mins |
Lesson proper
|
- Develop the lesson/ concepts through the use of pictures (See Attachment 6.F.3.2) that show the different uses of water in our daily lives.
- Let the learners discuss the pictures. Ask them how we can conserve water based on the pictures.
- Group the learners. Let the learners discuss the reasons why there is a scarcity of water and the ways to solve this. Place the answers using the matrix below.
|
35 mins
|
|
Reasons why water is scarce |
Ways to solve it |
|
|
|
|
Table 6.F.3.1. Ways to solve water scarcity |
|
|
- Group the learners again. Ask them to discuss the importance of water in our daily lives and other ways by which we can conserve it. Use the matrix below to present their answers.
Table 6.F.3.2. Water problems and ways to conserve |
|
|
Importance of water |
Problems about water |
Ways to conserve water |
|
|
|
|
|
|
|
|
|
- Let the learners present their work through reporting.
|
|
Closure |
Learners in their respective groups should do the following:
- Ask the learners to work in groups and make a poster or a slogan with these themes:
- Water conservation
- Planting trees help maintain the water cycle and preserve water sources.
- Ask the learners to write an essay individually about the importance of water in our daily lives.
|
30 mins.
25 mins. |
Assessment / Evaluation |
Observation and test (See Attachment 3) |
5 mins |
Attachment 6.F.3.1
Song of Tubig ay Tipirin

Attachment 6.F.3.2
 The uses of water The uses of water


Attachment 6.F.3.3
Assessment / Evaluation (5 mins.)
A. The learners will take a short quiz based on the lesson.
Direction: Write TRUE if the statement is correct. Write FALSE if the statement is wrong.
____________1. Water is life.
____________2. Throwing dead animals in the river makes the water polluted.
____________3. Turn off the faucet while brushing your teeth.
____________4. Water is everywhere so we should not conserve it.
____________5. Waterways can be a good way to transport goods and people.
B. The learners will be given the Observation Checklist to be filled-up by their parents.
Observation Checklist
Place a check (a) if the child is doing the different ways of conserving water.

F.4. Water in culture, traditions and religious practices
F.4.1. Overview and Background Information
Water is a basic need of living things. Without water how could people exist? It is a necessity of life, thereby valuable attention and care should be given for the proper use of water. But because of the daily changes brought about by the processes of manufacturing of different products, automation of machines and appliances and food processing the physical environment is greatly damaged, particularly the water system, due to improper management of garbage disposal.
To alleviate the pain of sickness as an effect of the changes in the quality of drinking water, this lesson will educate young learners on the proper ways of throwing their waste materials to avoid contamination of water and for malpractices to be corrected.
F.4.2. The Lesson Plan
Subject Matter area |
Science and Health |
Time duration |
Topic |
Using Physical Change |
|
Sub-Topic |
Distillation |
|
Grade Level |
5 |
|
Specific concepts covered in the lesson |
- Distillation involves reversible stages of phase change. Distilled water, for example, is made by vaporizing water into steam and then condensing the steam back to water. The distillation of only one substance is called simple distillation.
- Distillation is a method of retrieving a pure substance from a mixture. It is one way of extracting, for instance, the essence of flowers in the production of perfumes and colognes.
- Distillation is a method of retrieving a pure substance from a mixture. It is one way of extracting, for instance, the essence of flowers in the production of perfumes and colognes.
- Simple distillation of water is nature’s way of maintaining balance in the environment. This is called the water cycle. Liquid water is vaporized through the combined action of evaporation and transpiration. Water vapor forms clouds. When the clouds are cooled, these condense back to water and fall as rain.
- Distillation is also used to separate a mixture of different liquid substances. Since liquid substances have different boiling points, these can be separated from their mixtures through distillation at different temperatures. This is called fractional distillation.
|
|
Human values addressed |
- Right Conduct
- Maturity
- Raising our Standard of living
- Truth and Honesty
- Self-discipline and Cleanliness
- Fear of God
- Thriftiness and Economy
- Awareness in water conservation
|
|
Lesson objectives |
To describe how water is purified using physical change to improve the environment.
To use physical change to protect and improve the environment.
To relate distillation of water to purification of oneself as a sign of maturity. As we mature, we learn many things and we change for the better. |
|
Materials needed |
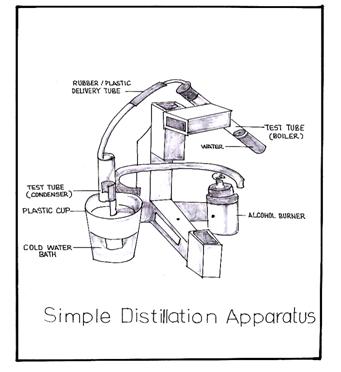
- Activity materials: simple distillation apparatus, colored water, salt, alcohol burner
- Reading materials
- Activity Worksheet
- Evaluation sheets
|
|
Teaching-Learning Procedures: |
90 min |
Introductory or Motivational activities |
Checking of Attendance
Checking of Assignments |
5 mins
|
Pre-activity |
By asking:
- What is the boiling point of pure water?
- How does a solute affect the boiling point of water?
- Set standards to be employed during the activity proper.
- Briefly review on the precautions and laboratory techniques.
|
5 mins |
|
There was a time when men drink from the rivers and lakes. When population increased, so did pollution. The rivers and lakes became the catch basins of water. Then the water pump was invented. (Demonstrate a lift pump or force pump.) Water from the pumps was more potable because it was filtered through layers of clean soil. But these advances in science and technology caused more pollution until water from the pumps became less and less potable. New methods of water purification were invented until someone thought of bottling mineral and distilled water. Today, more and more people are drinking bottled water.
By asking:
- How many of you are drinking bottled water?
- How many glass of water do you drink daily?
- Is it safe to drink water from the rivers and lakes?
- How can you help maintain the cleanliness and purity of water to improve the environment?
Through modern technology, mineral and distilled water are now available everywhere to supply our need for potable water |
10 mins |
Lesson proper
|
- Distribute to the learners the reading material about “Using Physical Change.” (Attachment 6.F.4.1)
- Let the learners read the material silently before starting the discussion about it. Remind them the Standard for Silent Reading.
- Sit well.
- Hold the reading material with both hands.
- Read with your eyes and do not move your lips.
- Do not point to the word.
- Do not move your head.
- Understand what you read.
- Explain that distillation involves reversible stages of phase change. Ask: How is water distilled?
- Say that this type of distillation is called simple distillation because it involves the distillation of only one substance.
- Simple distillation of water is nature’s way of maintaining balance in the environment. This is called water cycle. Describe the water cycle.
(Answer: Water cycle is a physical process involving a number of phase changes. It is the process by which water in the oceans or land evaporates into the air, is transported by winds, condenses as rain and the other forms of precipitation, nourishes the land, and eventually returns to the sea, in the rivers and groundwater.)
- Distillation is also used to separate a mixture of different liquid substances. Since liquid substances have different boiling points, these can be separated from their mixtures by distilling them at different temperatures.
Ask: What is this distillation called?
(Answer: Fractional Distillation)
- In your activity, you will distill water with the use of a simple distillation apparatus.
|
10 mins |
Enhancement Activity:
- Distribute the activity worksheets. (See Learners Activity Worksheet - Attachment 6.F.4.2)
- Perform the activity for enrichment.
|
45 mins |
Linkage with human values: |
- A person learns to use water wisely in order to practice thriftiness and economy. This is a sign of maturity. When the proper use of clean water becomes a habit, it may raise our standard of living.
- In our lives, we also undergo purification as we change from one state to another. This is the maturing process. We learn as we grow and we change for the better. We try to be God-fearing, by practicing truth and honesty just like paying our monthly water bills wisely and religiously. By doing so, we raise our standard of living.
- Water is pure. Let us maintain the purity of water .Have a pure mind, so that we can share and sustain the purity of water.
- By asking:
- How do you show your concern to water?
- How can you help conserve water?
- As a matured individual, how can you relate distillation of water in your daily living?
|
10 mins |
Closure |
Teacher emphasize about the use of distillation for purifying water |
2 mins. |
Assessment / Evaluation |
Evaluation:
Every learner is provided with a separate evaluation sheet of the test to be given. This will measure their achievement level of the day’s lesson. (Attachment 6.F.4.3)
Assignment
Read the lesson about Purification of drinking water.(Attachment 6.F.4.4) |
8 mins |
Attachment 6.F.4.1
Using Physical Change
“Distillation”
Distillation involves reversible stages of phase change. Distilled water, for example is made by vaporizing water into steam and then condensing the steam back to water. This type of distillation is called simple distillation because it involves the distillation of only one substance. It is method of retrieving a pure substance from a mixture. Distillation is one way of extracting the essence of flowers in the production of perfumes and colognes.
Simple distillation of water occurs in nature as nature’s means of maintaining a balance. This is called the water cycle. Liquid water is vaporized by the combined action of evaporation and transpiration. Water vapor forms clouds. When the clouds are cooled, they condense back to water and fall as rain.
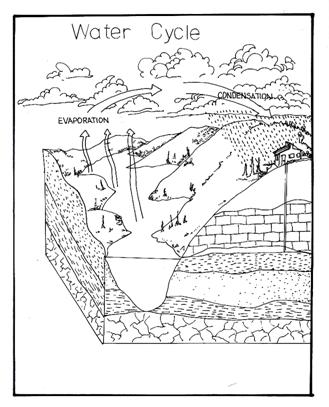
Distillation is also used to separate a mixture of different liquid substances. Since liquid substances have different boiling points, they can be separated from their mixtures by distilling them at different temperatures. This is called fractional distillation.
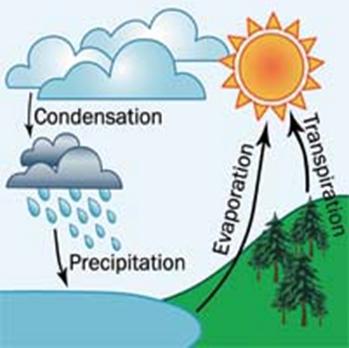
Water Cycle
Attachment 6.F.4.2
Activity Worksheet
Name : ____________________________
Grade : ____________________________
School : ____________________________
Topic : ____________________________
Sub-Topic : ____________________________
Activity : ____________________________
Problem : How is water purified by physical means?
Objective(s) of the activity:
- To purify water using the distillation method
Process and Steps:
Process |
Steps |
Note: time, etc. |
Preparation for learners to participate in the activities
- Group work set up
- Check learning materials
|
- Learners in groups with about 5-6 members each group
- Choose leader of group
- Checking your materials as below:
- Simple Distillation Apparatus
- Colored Water
- Salt
- Alcohol burner
- Reading materials
- Activity Worksheet
- Evaluation sheets
|
- in classroom or laboratory
- 5 minutes
|
Activities:
Step by step guidance for learners (incl. questions, and expected answers)
- Group work to purify water by distillation |
The tasks : Purify water using the distillation method:
- Fill one-third of the test tube boiler with colored salt water. Immerse the condenser test tube in a plastic cup of cold water. Plug the rubber stopper of the delivery tube into the boiler and insert the glass tubing into the condenser.
- Heat the colored salt water to boiling
- Observations and Inferences:
- What is the color and taste of the water that drips from the glass delivery tube?
- What can you infer from these observations?
- What phase changes take place in distillation?
- Why is distillation a physical change?
- Generalization
|
- in laboratory
- 25 minutes
|
Expected Results
- The results of experiment
- Presentation |
Present the result of experiments and answer the questions |
- in classroom or laboratory
- 15 minutes
|
Answers of Activity Worksheet
Not all the answers are given here. Where your child is asked to give an opinion or idea we suggest these are checked by you. Some answers are given as examples and are not intended as the only correct response.
- The water that drips from the delivery tube of the simple distillation apparatus is colorless and tasteless.
- I can infer that distilled water is made by vaporizing water into steam then condensing the steam back to water.
- Evaporation and condensation are the two-phase changes that take place in the distillation of water.
- Distillation is only a physical change because the water did not change its composition. No new substance is formed.
Generalization:
Water is purified by distillation .Distillation is a physical means because it does not involve any change in the composition of matter. In other words, no new substance results from the physical means.
Attachment 6.F.4.3
Evaluation Sheet
Name : _______________________________
Grade : _______________________________
School : _______________________________
Topic : _______________________________
Sub-topic : _______________________________
Fill each blank with the right answer
- The two processes involved in simple distillation of water are _____________________ and ___________________.
- Simple distillation involves _______________________ substance in the Purification of water.
- The phase change that take place in the distillation of water is ________________ change because no ____________ is formed.
- Water is purified by _____________ when it involves reversible stages of phase change.
- Distilled water is made by _______________ water into steam then _____________________ the steam back to water.
Answers of Evaluation Sheet
Not all the answers are given here. Where your child is asked to give an opinion or idea we suggest these are checked by you. Some answers are given as examples and are not intended as the only correct response.
- evaporation and condensation
- one
- physical – new substance
- distillation
- vaporizing- condensing
Attachment 6.F.4.4
Purification of drinking water
Water pollution in most areas in the Philippines is caused largely by the general public in the form of domestic garbage and sewage. The absence of strict measures to stop this kind of pollution has seriously compromised the portability of our drinking water.
Fortunately, the soil has an amazingly effective means of filtering suspended particles. But no matter how effective its filtration capabilities, a continuous build up of pollutants can still damage the water ecosystem. Some kind of purification system must be adapted to insure safe water for generation to come.
To make water potable, it is treated in three stages. The first stage, or primarily treatment consists of removing solid wastes from sewage and allowing the water to filter through the ground. In many areas, the primarily treatment called cesspool method, is the only method used in purifying drinking water. A cesspool is a large tank dug in the ground. Sewage flows from the house into the cesspool. In urban areas where government and NGO support is available, a more scientific method of primarily treatment is employed. Sewage is introduced into a sedimentation tank through a screen, which filter the solid wastes. In the sedimentation tank, much of the suspended particulates settle down at the bottom. The water is then introduced into a coagulation tank, where most of the remaining particulates are coagulated by the addition of chemicals. Then the sewage enters a centrifugal pool where even the finely divided particles are removed.
From the centrifugal pool, the water is ready for secondary treatment. This involves the use of bacteria to breakdown toxic waste. This is done by passing through a biological filter bed, or gravel that has been seeded with bacteria. Finally, water is chlorinated. Chlorine is used in the tertiary treatment in order to kill the disease-causing germs that have not been eliminated by the primary and secondary treatments. |
















